Veterinary vaccines are mainly composed of either subunit or inactivated bacteria/viruses. These vaccines require optimal adjuvants (except for DNA vaccines) and have various benefits.

Veterinary vaccines are mainly composed of either subunit or inactivated bacteria/viruses. These vaccines require optimal adjuvants (except for DNA vaccines) and have various benefits, including dose reduction, greater efficacy in the elderly, and a broader immune response.
Veterinary vaccines adjuvants
Subunit recombinants are still more likely to be absorbed than pathogens that have been inactivated or live attenuated. They are, however, generally less immunogenic and require an adjuvant to stimulate the immune system to combat the disease. Numerous adjuvants, including mineral salts, have been evaluated for use in veterinary vaccines (aluminum), emulsions (Montanide), and polymeric micro-and nanoparticles that degrade in the body.
In addition, “immune potentiator” refer to adjuvants that directly affect immune cells, resulting in activation of the immune system.
In Pakistan, mostly inactivated vaccines include Bacterins of one or even more bacterial strains or dead viral strains produced most frequently in an oil or aluminum hydroxide adjuvant. Inactivated vaccines are more stable and cost less than live vaccines.
Typically, the vaccine virus is grown in cell culture in roller bottles or bioreactors. Inactivation of the pathogenic organism to produce killed vaccines is achieved through physical or chemical procedures that result in protein denaturation or nucleic acid damage. Further purification of inactivated antigen and adjuvant mixing is done.
There is an urgent need to develop new and improved veterinary and human vaccine adjuvants.
Haemorrhagic Septicaemia
Haemorrhagic Septicaemia (HS), locally known as Gal-Ghuto, is a significant epizootic disease that is fatal and highly contagious caused by the Veterinary pathogen Pasteurella multocida (P. multocida) serotypes B:2 in Asia and E:2 in Africa. P. multocida is a pleomorphic coccobacillus bacterium, bipolar staining, gram-negative, and non-motile bacteria and its infections in humans are rare.
HS commonly occurs in both cattle and buffaloes, but buffaloes are more susceptible than cattle, and the disease occurs more frequently in developing countries due to poor husbandry conditions and causes significant economic loss.
Haemorrhagic Septicaemia is included in the Old Classification of Diseases Notifiable to the OIE List B, which is transmissible diseases that are considered to be of socio-economic and public health importance within countries and that are significant in the international trade of animals and animal products.
HS caused a loss of USD 130,000 in India between 2007 and 2011, while more than half a million dollars are lost each year due to this disease in Malaysia. Cattle and buffalo populations in Pakistan were expected to have reached 39.7 million and 34.6 million, respectively, during the years 2013–2014. Researchers found that 20 million dollars had been lost per year in Pakistan due to the HS outbreak.
Clinical Signs
Clinical symptoms are often not observed due to the acute nature of the disease but include high temperature(1040-1080F), loss of appetite, nasal discharge, increased salivation and laboured breathing, with the subcutaneous edema of the mandible, neck, and brisket is the distinctive features of the disease.
Death usually occurs quickly, and mortality is virtually 100% in infected animals. Proper recovery from clinical disease occurs only if the animal is treated in the very early stages, which is often impossible under prevailing field conditions.


Postmortem lesions
Upon opening an animal carcass that has died of HS, the most apparent lesion is subcutaneous edema-subcutaneous infiltration with yellow serosanguinous fluid, particularly in the submandibular and brisket regions. Subcutaneous petechial haemorrhages are also evident.
There are also widespread petechial haemorrhages in the thoracic cavity, particularly on the base of the ventricles and the auricles. There may be excessive fluid in the pericardial sac, and pericarditis with marked thickening of the pericardial wall may be present. The lungs may be congested with varying degrees of consolidation and with a marked thickening of the interlobular septa.
In the abdominal cavity, petechial haemorrhages are widespread in all tissues. Massive ecchymotic or petechial haemorrhages may also begin to show during this phase. As the disease progresses, the nasal discharge becomes opaque and mucopurulent. The respiratory distress becomes more acute, the animal lies down, and fatality is nearly 100%.
Figure 3: Haemorrhages on Trachea
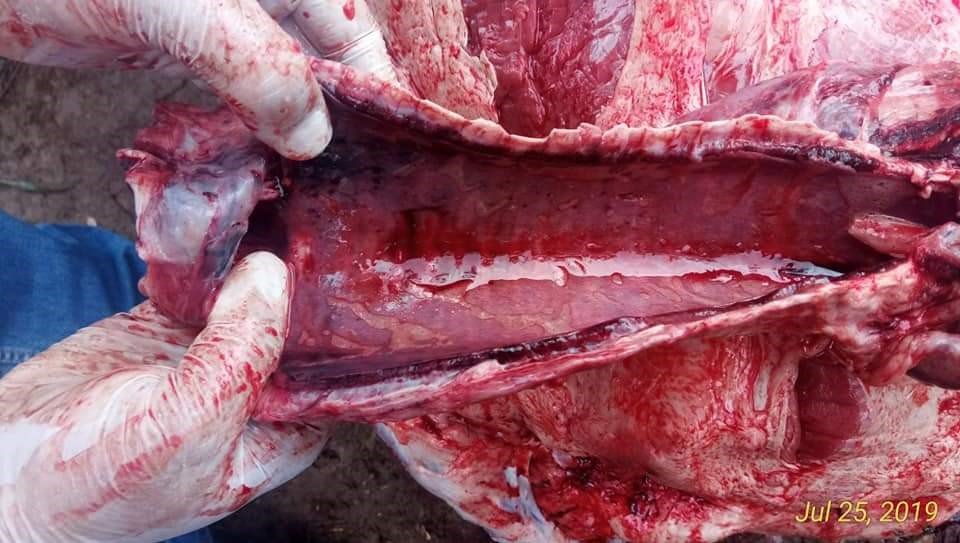
Figure 4: Congested Lungs

Figure 5: Coalescing Petechiae on Epicardium
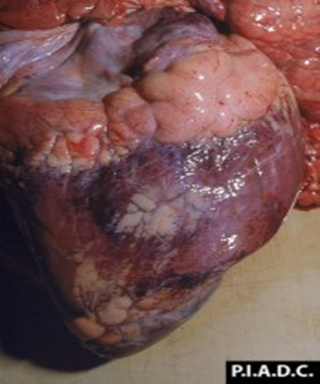
Figure 6: Submandibular Edema

Treatment
Antibiotics are effective only if they are started very soon after the onset of clinical signs. A common practice is to monitor animals for fever and treat febrile animals immediately during outbreaks. Some drugs used to treat haemorrhagic septicemia include oxytetracycline, trimethoprim/ sulfamethoxazole, a combination of penicillin and streptomycin, or sulphaquinoxaline. Antibiotic resistance has been reported in some endemic areas.
The primary reasons for treatment failure in infected animals are the acute nature of the disease and its shorter duration. Only a comprehensive vaccination campaign will halt the disease’s spread, and Humoral immunity is essential for disease defence. Vaccination is more effective than almost any measure of reducing mortality in HS.
HS Vaccines
Three of the most popular vaccines in Asia are the whole-cell formalin killed (Bacterins) P. multocida oil Adjuant vaccine; Alum (adjuvant) precipitated vaccine, and Aluminium hydroxide gel vaccine
Aluminium hydroxide gel vaccine
Countries such as Laos and Thailand have been heavily involved in the development of an aluminium hydroxide gel vaccine and claimed that even with the use of supportive medicines or the body’s immune modulators like vitamin E and levamisole, this type of vaccine could not provide significant immunity for more than 90 days.
Alum (Adjuvant) precipitated vaccine
Before the 1950s, commercial manufacturers in the United States and the Philippines appeared to have experimented with alum-precipitated vaccines. Southern India and Central Africa have seen widespread use of the vaccine Alum (adjuvant) precipitated vaccine immunity induced by this kind of vaccine lasts for only 3-4 months.
Oil Adjuvant vaccine
A local strain of P. multocida is used to make an oil adjuvant vaccine (OAV). Formalin is used to cultivate and inactivate the strain. Centrifugation is used to generate bacterial pellets, which are then adjuvanted with Montanide oil ISA-50.
Commercially available HS Vaccines in Pakistan
Both Alum (Adjuvant) precipitated vaccine and Oil Adjuvant (Montanide) vaccines are prepared by Veterinary Research institute Lahore (VRI) and commercially available in Pakistan.
Figure 7: Commercially available HS vaccines

Novel Eolane Based HS vaccine
Novel EOLANE is a mineral oil TOTAL Special Fluid company that claims that Eolane oil is a Short-chain hydrocarbon (C15– C20) with Low viscosity, Low PAH content and is biodegradable. As an adjuvant in vaccines, this oil has high efficacy, safety and is easy to inject.
Novel Eolane Based HS vaccine produced under Punjab Agriculture Research Board (PARB) funded Project No. 629 titled “Development 0f Cost-Effective Oil-Based Haemorrhagic Septicemia Vaccine Using Eolane as Adjuant” project awarded to Prof. Dr Syed Ehtisham-ul-Haque Chairman Department of Pathobiology CVAS Jhang and Dr Waseem Shahzad Sr. Veterinary Officer Veterinary Research Institute (VRI), Lahore, Pakistan.
The master seed for HS vaccine prepared by VRI and preparation of Bacterins (killed bacterial vaccine) antigen suspension was prepared as 0.5% formalized seed culture and adjusted concentration as 2mg/ml suspension. Final vaccinal preparations were made by mixing antigen suspension with mineral oils as 1:1 Oil adjuvants Eolane-170 (E170) and Eolane-150 (E150) is used.
Novel Eolane based vaccines are prepared and successfully evaluated in lab animals at VRI and University of Veterinary and Animal Sciences Lahore (Sub-campus Jhang). After a successful evaluation of immune response in large animals (buffalo and cattle calves), by Indirect Haemagglutination (IHA) and by specialized in-house prepared indirect enzyme-linked immunosorbent assay (ELISA) kit by comparing the efficacies with commercially available and imported vaccines, the best Eolane Based vaccine will be available soon to the farmers.
The Montanide-based vaccination is currently being used in Pakistan to combat HS. Adjuvant Montanide must be imported from France at a high cost, even though the vaccination is effective for up to one year. Vaccine production may be hampered in an emergency if supplies are delayed. It is, therefore, necessary to have an adjuvant as an alternate and cost-effective and immunogenic for the entire year. It will revolutionise advancements in veterinary vaccine adjuvants and develop a cost-effective, safe, Novel Eolane based HS vaccine.
This article is jointly written by: Abdul Subhan*1 Aman Ullah Khan1, Syed Ehtisham-ul-Haque1, Muhammad Fiaz Qamar1, Muhammad Adnan Saeed1, Waseem Shahzad2, Tyyba Arshad1, Shanza Khan1, Muhammad Saqlain1, izza3, 1Department of Pathobiology, University of Veterinary and Animal Sciences, Lahore (CVAS, Jhang Campus), 2 Veterinary Research Institute, Lahore, Pakistan, 3 Institute of Microbiology,University of Veterinary and Animal Sciences, Lahore
