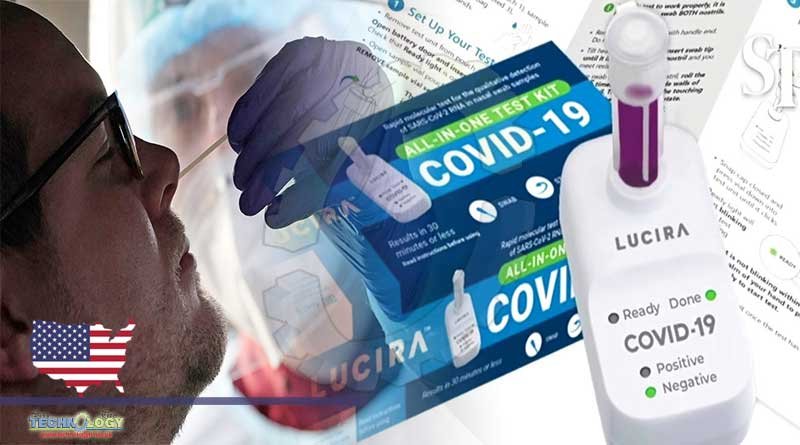
One can now take a COVID-19 test in the comfort of his home and get results in just 30 minutes with the FDA authorized take-home rapid tests.
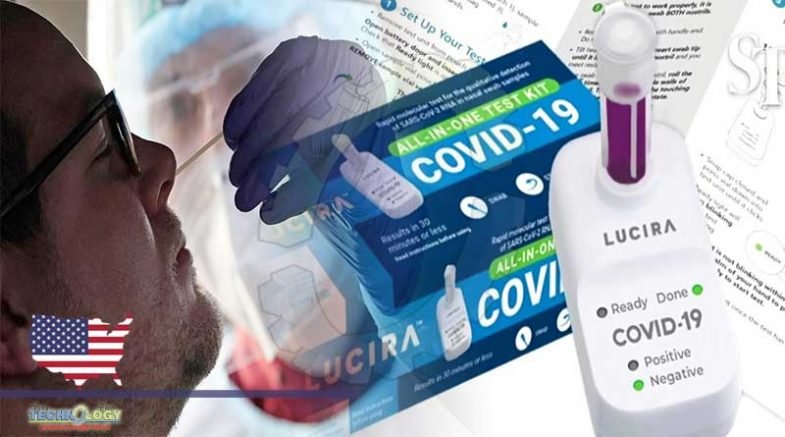
One can now take a COVID-19 test in the comfort of his home and get results in just 30 minutes. This latest development comes after the Food and Drug Administration (FDA) has authorized the three companies’ take-home rapid tests.
Specifically, according to a VICE News report, Ellume, Abbott and Lucira’s diagnostic tests are “much more accessible” compared to the laboratory polymerase chain reaction or PCR tests, which need professional equipment and “can cost hundreds of dollars.”
The technology applied in most of these take-home rapid tests is surprisingly akin to a home pregnancy test kit and, the said report specified that they’ll be priced below $50.
With fast-response tests, a user simply applies a nasal swab sample to a sample testing device. Then, within roughly 30 minutes, a result will indicate if an individual testing is positive for COVID-19.
Take-Home Rapid Tests
The take-home rapid tests are pieces of papers covered with a substance designed to detect COVID-19 antigens, coating the virus, and inducing an immune system response.
Therefore, if a person is infected with the virus at the very moment, the test is likely to come back positive. Once an individual becomes infection-free, they are not likely to carry any antigens anymore.
However, if one exposed to COVID-19 took the test too early or before they get an adequate viral load, the result may come back with a negative result.
Still, rapid tests are designed to help individuals catch an infection on a daily basis, not just at a time they feel ill, and those in medical practices are hoping to shift testing’s social paradigm altogether.
In an email to the news site, Washington DC-based travel precaution specialist, Dr. Ida Bergstrom said, if one just gets a PCR if he has symptoms or is exposed, “then you will find out if you already have COVID-19.”
However, that depends on typically waiting for the test, and then another two to three days or sometimes longer, for the result, and quite a few people are quarantining during that time, explained the doctor.
COVID-19 Tests’ Accuracy
Many other companies are seeking approval from the FDA for their rapid diagnostic tests for COVID-19 that would cost below $10.
Small private firms, including E25Bio, and even big players in the industry like 3M have teamed up with schools such as Harvard and MIT to acquire approval to start distribution at a large scale.
In September 2020, Nature came out with a report stating that PCR tests are “95 to 100 percent accurate all of the time.
According to a Harvard Medical School article, both PCR and antigen tests, the said report specified that false positives need to take place “around zero percent of the time.”
Meanwhile, rapid-response antigen tests can be 75-percent accurate if an individual gets tested more than one week from coming down with the contagion.
Within the first week, the figure jumps from 84 to 95-percent accurate, the 2020 report specified. However, these rapid COVID-19 tests are not meant to provide a definitive answer.
Rather, their ultimate goal, which one of the three tests presently allows, is to let individuals monitor themselves throughout this pandemic, regardless of the symptoms.
The United States is not the only country that authorizes take-home rapid tests. South Korea, in particular, has made them widely available to consumers, although there is some concern when it comes to their accuracy.
Rapid diagnostic tests are being used as well in developing countries where healthcare and access to laboratories capable of conducting PCR testing are limited.
All three take-home rapid COVID-19 tests are from Ellume, Abbot and Lucira and are authorized by the FDA in the US. They all cost roughly $50.
Originally published at The Science Times