Ebola virus is a negative-sense ssRNA virus belonging to the family Filoviridae, which is enveloped, pleomorphic, and replicates in the cytoplasm.
Ebola virus is a negative-sense ssRNA virus belonging to the family Filoviridae, which is enveloped, pleomorphic, and replicates in the cytoplasm. The Ebola virus, also called the Zaire Ebola virus, is the most lethal of the Ebola viruses.
The Ebola virus, also called a bioterrorism agent, is known to cause highly lethal hemorrhagic fever. It first appeared in 1976 in two simultaneous outbreaks, one in what is now Nazara, South Sudan, and the other in Yambuku, Democratic Republic of the Congo. The latter occurred in a village near the Ebola River, from which the disease takes its name, the Ebola virus.
The mortality rate can be as high as 90 to 95%. Because the Ebola virus is so hazardous, it is classified as a biosafety level 4 agent. Ebolavirus species that are associated with humans are the Ebola virus, Bundibugyo Ebola virus, Sudan Ebolavirus, Tai Forest Ebolavirus, and Reston Ebolavirus. Reston Ebolavirus was first discovered in crab-eating macaques (monkeys) in a laboratory in Reston, Virginia, in the United States, and appears not to be pathogenic to humans.
Transmission:
Bats of the Pteropodidae family are natural Ebola virus hosts. Ebola is entered into the human population through close contact with the blood, secretions, organs, or other bodily fluids of infected animals such as fruit bats, chimpanzees, gorillas, and monkeys.
Ebola then spreads through human-to-human transmission via direct contact (through broken skin or mucous membranes). Blood or body fluids (urine, saliva, sweat, faeces, vomit, breast milk, amniotic fluid, and semen) of a person who is sick with or has died from Ebola disease, Objects (such as clothes, bedding, needles, and medical equipment) contaminated with body fluids from a person who is sick with or has died from Ebola disease and Burial ceremonies that involve direct contact with the body of the deceased can also contribute to the transmission of Ebola.
A person can only spread an Ebola virus, also called the Zaire Ebola virus, to other people after they develop signs and symptoms of the disease. Its incubation period is 2–21 days.
Viral protein of the Ebola virus:
The genome of the Ebola virus is 19 kb long, with seven open reading frames encoding structural proteins, including the virion envelope glycoprotein (GP), which helps in attachment, nucleoprotein (NP), and matrix proteins VP24 and VP40; nonstructural proteins, including VP30 and VP35; and the viral polymerase, VP40, which helps to maintain structural integrity.
It has also been associated with endosome formation and likely mediates filovirus budding due to its ability to induce its own release from cells in the absence of all other viral proteins, and VP24 inhibits interferon production.
This protein, along with VP35 and NP, is sufficient to form nucleocapsid structures, and VP24 is necessary for the correct assembly of a functional nucleocapsid. The viral-specific transcription activator vp30 and the viral RNA polymerase.
Pathogenesis of the Ebola virus:
- Zaire Ebola virus enters a person through mucous membranes, breaks in the skin, or parenterally and infects many cell types, including monocytes, macrophages, dendritic cells, endothelial cells, fibroblasts, hepatocytes, adrenal cortical cells, and epithelial cells. The GP is the surface protein able to interact with cellular receptors: a sialoglycoprotein receptor on hepatocytes, the folate receptor α on epithelial cells, and c-type lectins, present in dendritic cells (DC), macrophages, and endothelial cells.
- The incubation period may be related to the infection route. The Ebola virus is migrated from the initial infection site to regional lymph nodes and subsequently to the liver, spleen, and adrenal gland.
- Although not infected by the Zaire Ebola virus, lymphocytes undergo apoptosis, resulting in decreased lymphocyte counts. Hepatocellular necrosis occurs and is associated with dysregulation of clotting factors and subsequent coagulopathy.
- Ebolaviruses appear to trigger a release of pro-inflammatory cytokines with subsequent vascular leakage and impairment of clotting, ultimately resulting in multiorgan failure and shock.
- Host immune responses to the Zaire Ebola virus and cell damage due to direct infection of monocytes and macrophages cause the release of cytokines associated with inflammation and fever (A). Infection of endothelial cells also induces a cytopathic effect and damage to the endothelial barrier that, together with cytokine effects, leads to the loss of vascular integrity.
Signs and symptoms:
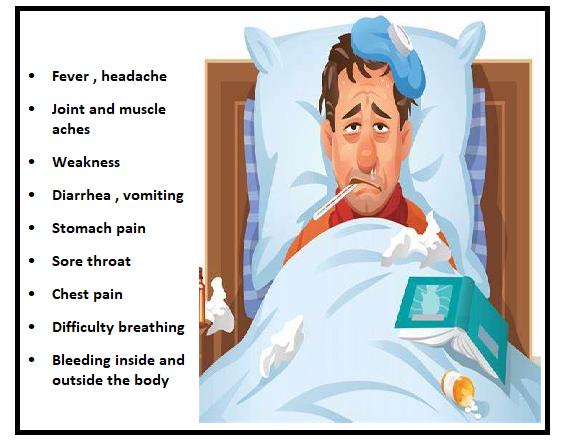
Signs and symptoms of the Zaire Ebola virus are shown in Figure 1.
Management and Diagnosis of the Ebola Virus:
EBOV is categorised as a high-hazard pathogen that is handled at biosafety level 4; thus, it should be managed in level 4 facilities (BSL4). These laboratories (BSL4) have the following main characteristics: high-efficiency particulate air (HEPA) filtering, air-handling systems with negative pressure, all waste material is inactivated, and there is a chemical shower for worker decontamination.
Staff are highly skilled and trained for years before working at this level of containment. Moreover, mobile diagnostic laboratories could represent a facility to carry out a rapid diagnosis. In a mobile laboratory, there are three separate areas, which include places for inactivation and extraction, reagent preparation, and quantitative reverse-transcription polymerase chain reactions (RT-PCR).
Sample Management:
Samples should be sent to a lab with facility level 4 in appropriate packaging (UN2814 [category A]). WHO recommendations for venipuncture in cases of suspected EBOV state that blood should be collected in EDTA tubes with a minimum volume of 5 ml.
The WHO guidelines further state that blood samples can be stored for up to 24 hours at room temperature or at 0–5 °C for up to a week. For periods longer than a week, the sample should be stored at −20◦C or −70◦C.
Ebola virus inactivation:
Commonly used disinfectants include 3% Lysol, 5% Microchem Plus, and 0.5% hypochlorite solutions. The addition of ethanol to the procedure is necessary to ensure the full inactivation of samples.
Different Tests for Diagnosing the Ebola Virus:
- CBC (Complete Blood Count)
- ELISA tests for specific Ebola proteins.
- Immunofluorescent assay: the patient develops IgM and IgG antibodies.
- RT-PCR.
- rapid diagnostic test.
Rapid diagnostic test:
The difficulty in accessing an early and fast diagnosis that guarantees efficient contact tracing and isolation of EVD-positive patients limits the control of epidemics. It has been hypothesized that the use of RDTs with a specificity and sensitivity of 99% could reduce the number of cases in the Sierra Leone epidemic by 42%.
Current molecular diagnostic methods such as the polymerase chain reaction require trained personnel and laboratory infrastructures, which hinder diagnostics at the point of need, particularly in outbreak settings, and frequently the samples should be sent to a reference center to be analysed, resulting in a delay in the availability of results.
Point of care Rapid diagnostic tests substantially reduce these delays. The WHO issued a call for rapid, sensitive, safe, and simple Ebola diagnostic tests strictly related to ASSURED indications: minimal laboratory facility, no cold chain, and can be performed with a capillary blood sample collected through a finger prick, with a test result in minutes rather than days. For vp40 protein, the sensitivity in plasma of DEDIATEST Ebola is 90.53%.
Differential diagnosis:
The symptoms observed in EVD patients are similar to those linked to other hemorrhagic fever infections: fever, myalgia, chills, and malaise. The non-specific symptoms of EVD pose a major problem for triage and isolation efforts at Ebola treatment centres, etc.
Viral hemorrhagic fevers (VHF) and malaria have overlapping clinical characteristics, making differential diagnosis a challenge. A new immunoassay has been developed called surface-enhanced raman scattering (SERS), which at the same time detects antigens from Ebola, Lassa, and malaria within a single blood sample. Results are provided quickly (<30 min) for each sample. To test.
For the performance of this assay, 190 Ebola-positive clinical samples, 163 malaria-positive samples, and 233 negative controls were used. The results showed a sensitivity of 90.0% and a specificity of 97.9% in Ebola detection. In malaria detection, the sensitivity and specificity were 100.0 and 99.6%, respectively. These results indicate the potential of the SERS technology as an important tool for outbreak management in low-resource settings.
Prevention:
- Washing hands properly.
- Avoid touching the body fluids of people who have, or may have, Ebola.
- Not touching the bodies of people who have died from Ebola or getting the Ebola vaccine if they are at risk for the Zaire type of Ebola.
- Reducing the risk of wildlife-to-human transmission reducing the risk of human-to-human transmission outbreak containment measures, including safe and dignified burial of the dead.
- reducing the risk of possible sexual transmission.
- reducing the risk of transmission from pregnancy-related fluids and tissue.
Epidemiology:
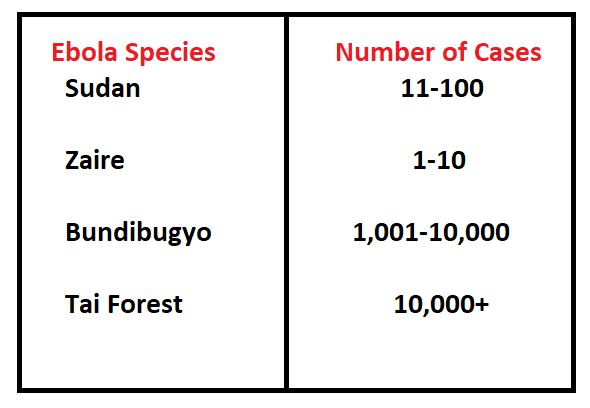
Ebola is not prevalent in Pakistan and is not recognised as an epidemic. The average Ebola case fatality rate is around 50%. Case fatality rates have varied from 25 to 90% in past outbreaks, depending on circumstances and the response. Reported cases of Ebola are shown in Figure 2.
This article is jointly authored by Muhammad Hamza, Aziz Ur Rehman, Usman Ali, Faisal Rehman, and Muhammad Hussain from the University of Veterinary and Animal Sciences Lahore, subcampus Jhang.