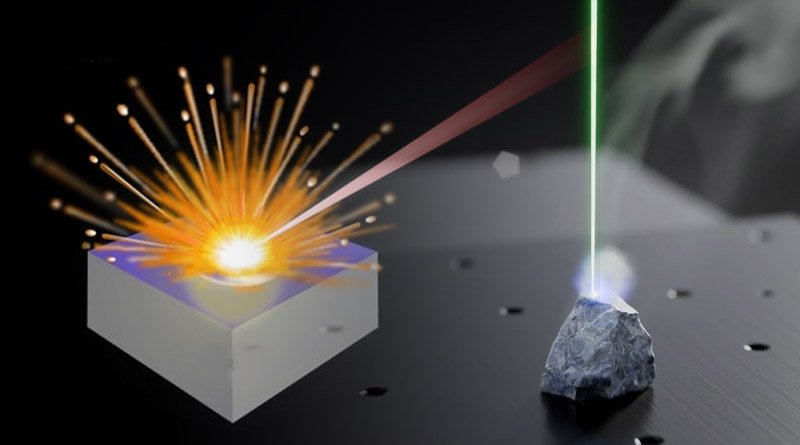
Among different kinds of emission spectroscopy, Laser Induced Breakdown Spectroscopy (LIBS) has become an efficient type of spectroscopy for analyzing all types of matter.
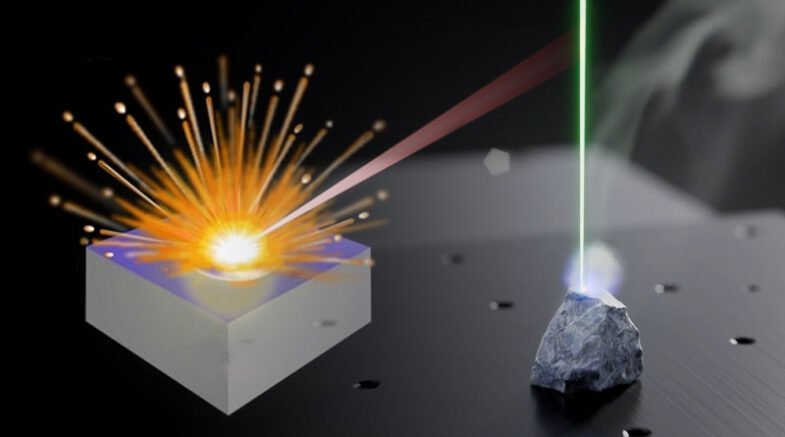
Among different kinds of emission spectroscopy, Laser Induced Breakdown Spectroscopy (LIBS) has become an efficient type of spectroscopy for analyzing all types of matter.
As we know that LIBS is emission spectroscopy, that uses a laser pulse (high energetic) for excitation and helps us to understand the quantitative and qualitative analysis of the matter. LIBS techniques are useful to identify the composition of the target material, its impurities, and the abundance of elements. LIBS has an edge over other analytic techniques because it is a rapid, efficient, and multi-elemental analysis.
LIBS, an efficient spectroscopy does not take a very long period for target analysis as compared to other techniques and a small amount of target is enough for detailed analysis.
In the LIBS technique, a laser pulse (Nd: YAG) is concentrated on samples (gases, liquid, or solid), and thus plasma appears on the sample surface at a very high temperature and a tiny amount of material is vaporized. .
When plasma expands the atoms of the sample are excited, these excited atoms effort to relax and resulting spectra is emitted. The spectrometer contains a prism and a camera to photograph the spectra for further study. LIBS spectra are utilized to identify different elements in the sample.
Each LIBS spectrum not only detects the quantities of all naturally occurring elements, as well as some isotopic ratios and details about the atomic structure of the target material. LIBS is a spot analytic technique that produces laser ablation craters that range in size from 30 to 400 µm, depending on the laser power, energy, wavelength, distance, and characterization of the material.
Due to the spot analysis nature of Laser Induced Breakdown Spectroscopy, it is feasible to investigate spatial changes in material composition as well as to average shots taken at various points on the materials to determine the bulk and nanomaterial composition.
LIBS has been employed for a variety of purposes because it is a rapid, efficient, and portable technique that requires no sample preparation that delivers instantaneous, on-situ analysis.
It is even being employed robotically on Mars. Applications may be divided into two major categories: chemical fingerprinting and elemental analysis. It is feasible to concurrently detect or investigate many elements since every naturally occurring element emits light in the usually observed wavelength range (200 – 1000 nm).
Numerous items, including rocks, minerals, fingernails, food products, air particle matter, and natural and polluted soils, have undergone elemental analysis.
LIBS spectra are especially helpful for addressing the issue by utilizing the spectrum as a chemical fingerprint or signature of material and compared with the spectrum database of known material.
This concept has been used in a variety of contexts, including the origin of gems and conflict minerals, the identification of viruses, bacteria, germs, and infection in the blood, the technical qualities of roadway aggregates, quality control in industrial processes like heat treatments, and many other circumstances.
The word Quantagenetics® was introduced by Materialytics, a LIBS-based material science firm, to investigate that each material has a distinct chemical and structural fingerprint that may be seen in a LIBS spectrum. The Quantagenetics® signature may be utilized to analyze the substance’s origin and history of materials by compared with known samples or materials.
LIBS, an efficient spectroscopy has three main limitations. Firstly, the laser doesn’t always interact with the sample in precisely the same way, which causes the first major shot-to-shot intensity changes.
A common solution to this is to average many spectra of the same sample. Secondly, the accuracy and precision of more conventional methods, such as ICP-MS or XRF, are not achieved by elemental analysis employing univariate or multivariate calibrations.
Finally, as with any spectroscopic approach, each LIBS technique generates spectra that are specific to that instrument, therefore the same material studied on a different LIBS equipment will yield somewhat different results. These inter-instrument discrepancies are significant enough to avoid mixing data sets from several instruments when employing LIBS spectra for provenance investigations.
For LIBS analysis, there is no sample preparation required; it is a point-and-shoot approach. Although LIBS mainly investigates the surface of materials, it is essential to make sure that a clean, representative surface is investigated.
Theoretically, there is no restriction on the size or shape of the material investigation. The maximum sample size may be limited by the sample chamber, which is a common feature of desktop computers and is enclosed in eye-safe plastic. The spot size, which is mostly governed by laser wavelength, and power, defines the minimal sample size.