A type of life support system for patients with serious heart or lung issues is ECMO. It is thought to be crucial in the treatment of COVID-19 patients who are critically ill.
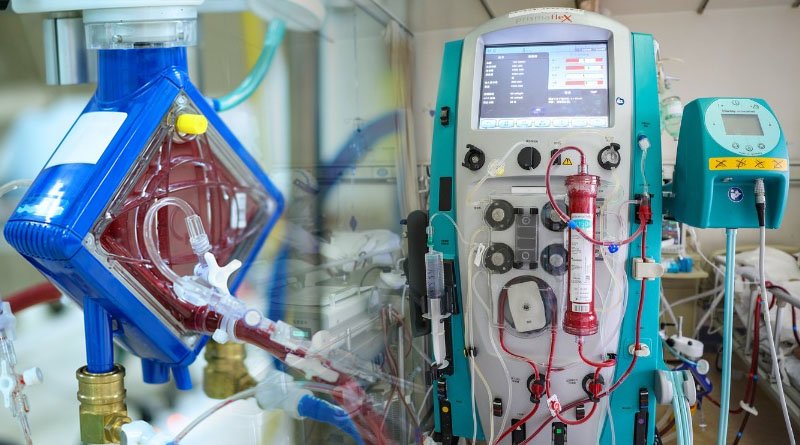
The official debut of the nation’s first extracorporeal membrane oxygenation (ECMO) device will be crucial in treating COVID-19 patients who are in critical condition and advancing the production of sophisticated medical equipment.
A type of life support system for patients with serious heart or lung issues is ECMO. It is thought to be crucial in the treatment of COVID-19 patients who are critically ill.
The first domestic ECMO apparatus, produced by Chinabridge Medical in Shenzhen, Guangdong Province, has been approved for emergency use by the National Medical Products Administration, China’s top drug regulator.
Key performance indicators for the product are comparable to those of its international competitors, the administration claimed in a statement. For China, the launch represents a significant advance in the design, research, development, and production of such high-end medical equipment, which has been dominated by foreign countries.
The Shenzhen Institute of Advanced Technology of the Chinese Academy of Sciences, Mindray, and Chinabridge Medical collaborated on the project as part of the National Innovation Center for Advanced Medical Devices.
According to Xin Guobin, vice minister of the Ministry of Industry and Information Technology, at the certificate-presenting ceremony in Shenzhen on Friday, the successful development closes a market gap in China and marks a significant turning point in the country’s development of high-end medical equipment.
Chinabridge Medical’s chairman, Liu Yang, claimed that rather than merely copying an item from abroad, the business has since 2018 concentrated on developing a product for clinical use with independent intellectual property rights.
The United States, Germany, and Japan are just a few of the developed nations where the company’s intellectual property rights have been approved. “This demonstrates the device’s being completely homegrown and innovative,” she said.
“A hospital like ours needs one ECMO unit for every 10 intensive care beds.” But as of yet, we haven’t achieved that standard. Therefore, the creation and application of such a medical device are urgently required, “proclaimed Geng Qingshan, president of the Shenzhen People’s Hospital, in charge of the ECMO clinical trials.
“The official release of the medical technology that was developed here in the country is a huge benefit for us.” “As soon as possible, we hope to use it on our patients.”