Here are two general classifications of covid-19 test: a viral test for a present disease, or a counter acting agent test for the past nearness of the virus.

The CDC doesn’t right now suggest testing for COVID-19 utilizing a CT output or searching for low oxygen levels.
The side effects communicated by COVID-19 patients are vague and can’t be utilized for an exact determination. Guan et al. announced that 44% of 1099 COVID-19 patients from China had a fever when they entered the medical clinic and that 89% built up a fever while in hospital.25 They further found that patients had a hack (68%), weariness (38%), sputum creation (34%), and brevity of breath (19%). A significant number of these manifestations could be related with other respiratory diseases. Nucleic corrosive testing and CT checks have been utilized for diagnosing and screening COVID-19.
Atomic methods are more reasonable than syndromic testing and CT filters for precise findings since they can target and recognize explicit pathogens. The advancement of atomic strategies is needy after comprehension the proteomic and genomic piece of the pathogen or the enlistment of changes in the outflow of proteins/qualities in the host during and after contamination. As of Walk 24, 2020, the genomic and proteomic creations of SARS-CoV-2 have been recognized, yet the host reaction to the infection is still under scrutiny. The main genome grouping of SARS-CoV-2 was directed with metagenomic RNA sequencing, a fair-minded and high-throughput strategy for sequencing various genomes.26−28 The discoveries were openly uncovered, and the succession was added to the GenBank arrangement vault on January 10, 2020.26,27 From that point forward, in excess of 1000 groupings have been made accessible on the Worldwide Activity on Sharing All Flu Information (GISAID) and GenBank by specialists over the globe.29,30 As per the joint report by the World Wellbeing Association (WHO) and China, 104 strains of the SARS-CoV-2 infection were secluded and sequenced utilizing Illumina and Oxford nanopore sequencing from the finish of December 2019 to mid-February 2020.2,4 Illumina sequencing is a succession by-blend technique utilizing strong stage connect enhancement, whereras nanopore sequencing includes translocating a DNA particle through a protein pore and estimating resulting shifts in voltage to decide the DNA sequence.31 Genome sequencing is significant for analysts to structure groundworks and test successions for PCR and other nucleic basic analyses.
Structuring a Nucleic Basic analysis for SARS-CoV-2
Nucleic corrosive testing is the essential technique for diagnosing COVID-19.32 various opposite transcription polymerase chain response (RT-PCR) packs have been intended to recognize SARS-CoV-2 hereditarily (Table 1). RT-PCR includes the opposite transcription of SARS-CoV-2 RNA into integral DNA (cDNA) strands, trailed by enhancement of explicit areas of the cDNA The structure procedure for the most part includes two primary advances: (1) grouping arrangement and preliminary plan, and (2) measure streamlining and testing. Corman et al. adjusted and broke down various SARS-related viral genome groupings to plan a lot of preliminaries and probes.35 Among the SARS-related viral genomes, they found three areas that had preserved arrangements: (1) the RdRP quality (RNA-subordinate RNA polymerase quality) in the open perusing outline ORF1ab locale, (2) the E quality (envelope protein quality), and (3) the N quality (nucleocapsid protein quality). Both the RdRP and E qualities had high expository affectability for recognition (specialized restriction of location of 3.6 and 3.9 duplicates per response), though the N quality gave less fortunate logical affectability (8.3 duplicates per response). The measure can be structured as a two-target system, where one groundwork generally recognizes various coronaviruses including SARS-CoV-2 and a subsequent preliminary set just distinguishes SARS-CoV-2.
Polymerase Chain Response (PCR) Tests/Introductions for SARS-CoV-2
In the wake of structuring the groundworks and tests, the subsequent stage includes advancing examine conditions (e.g., reagent conditions, hatching times, and temperatures), trailed by PCR testing. RT-PCR can be acted in either a one-advance or a two-advance test. In a one-advance measure, turn around transcription and PCR enhancement are united into one response. This examine arrangement can give fast and reproducible outcomes to high-throughput investigation. The test is the trouble in improving the opposite transcription and enhancement ventures as they happen all the while, which prompts lower target amplicon age. In the two-advance test, the response is done successively in isolated tubes.36 This examine group is more delicate than the one-advance measure, however it is additional tedious and requires upgrading extra parameters. Finally, controls should be painstakingly chosen to guarantee the dependability of the test and to distinguish exploratory mistakes.
Work process for Nucleic Corrosive Testing for SARS-CoV-2
In any event 11 nucleic-corrosive based strategies and eight neutralizer recognition packs have been affirmed in China by the National Clinical Items Organization (NMPA) for recognizing SARS-CoV-2. Be that as it may, RT-PCR is the most prevalently utilized technique for diagnosing COVID-19 utilizing respiratory samples. Upper respiratory examples are comprehensively suggested, in spite of the fact that lower respiratory examples are suggested for patients showing gainful cough.Upper respiratory tract tests incorporate nasopharyngeal swabs, oropharyngeal swabs, nasopharyngeal washes, and nasal suctions. Lower respiratory tract tests incorporate sputum, BAL liquid, and tracheal suctions. Both BAL and tracheal suctions can be high hazard for airborne age. The perceptible viral burden relies upon the days after disease beginning. In the initial 14 days after beginning, SARS-CoV-2 could most dependably be recognized in sputum followed by nasal swabs, though throat swabs were untrustworthy 8 days after side effect onset.Given the fluctuation in the viral burdens, a negative test result from respiratory examples doesn’t preclude the ailment. These negatives could result from inappropriate testing strategies, low popular burden in the territory examined, or changes in the viral genome. suggested various lines of proof for patients connected epidemiologically regardless of whether the outcomes are negative from nasopharyngeal or potentially oropharyngeal swab.
The US Habitats for Malady Control and Anticipation (CDC) utilizes a one-advance ongoing RT-PCR (rRT-PCR) measure, which gives quantitative data on viral burdens, to recognize the nearness of SARS-CoV-2.To play out the test, the viral RNA is separated and added to an ace blend. The ace blend contains sans nuclease water, forward and switch groundworks, a fluorophore-quencher test, and a response blend (comprising of converse transcriptase, polymerase, magnesium, nucleotides, and additives).The ace blend and extricated RNA are stacked into a PCR thermocycler, and the hatching temperatures are set to run the test. The CDC has suggested cycling conditions for rRT-PCR. During rRT-PCR, the fluorophore-quencher test is separated, creating a fluorescent sign. The fluorescent sign is recognized by the thermocycler, and the enhancement progress is recorded progressively. The test succession utilized by Guan et al. was Dark Opening Quencher-1 (BHQ1, quencher) and fluorescein amidite (FAM, fluorophore). This response takes ∼45 min and can happen in a 96-well plate, where each well contains an alternate example or control. There must be both a positive and a negative control to decipher the conclusive outcomes appropriately when running rRT-PCR. For SARS-CoV-2, the CDC gives a positive control arrangement called nCoVPC. various SARS-CoV-2 RT-PCR preliminaries and tests from various research gatherings and offices are recorded in Table 1.
Coordinating the Work process for Nucleic Corrosive Recognition with Clinical Administration
There are diverse execution work processes for RT-PCR tests in clinical settings. Corman et al. proposed a three-advance work process for the analysis of SARS-CoV-2. They characterize the three stages as first line screening, affirmation, and oppressive examines. To boost the quantity of contaminated patients distinguished, the initial step identifies all SARS-related infections by focusing on various locales of the E quality. On the off chance that this test is sure, at that point they propose the discovery of the RdRP quality utilizing two distinct preliminaries and two unique tests. On the off chance that these outcomes are additionally positive, at that point they direct the oppressive test with one of the two test sequencesChu et al. proposed a somewhat unique test workflow. They screened tests utilizing introductions for the N quality and utilized those from the ORFlb quality for affirmation. A finding where the patient example is sure with N quality groundwork and negative with the ORFlb quality would be uncertain. In such circumstances, protein tests (i.e., immune response tests) or sequencing would be required to affirm the diagnosis.
Figured Tomography
Because of the lack of packs and bogus negative pace of RT-PCR, the Hubei Area, China briefly utilized CT filters as a clinical conclusion for COVID-19. Chest CT examines are non-obtrusive and include taking numerous X-beam estimations at various edges over a patient’s chest to deliver cross-sectional images.48,49 The pictures are broke down by radiologists to search for strange highlights that can prompt a diagnosis.48 The imaging highlights of COVID-19 are assorted and rely upon the phase of contamination after the beginning of side effects. For instance, Bernheim et al. saw progressively visit ordinary CT discoveries (56%) in the beginning times of the ailment (0–2 days) with a greatest lung association cresting at around 10 days after the beginning of symptoms. The most well-known trademark highlights of COVID-19 incorporate two-sided and fringe ground-glass opacities (regions of murky opacity) and combinations of the lungs (liquid or strong material in compressible lung tissue). De Wever et al. discovered that ground-glass opacities are most unmistakable 0–4 days after side effect beginning. As a COVID-19 contamination advances, notwithstanding ground-glass opacities, insane clearing designs (i.e., sporadic molded cleared stone example) develop, followed by expanding union of the lungs. Dependent on these imaging highlights, a few review considers have demonstrated that CT examines have a higher affectability (86–98%) and improved bogus negative rates contrasted with RT-PCR. The primary proviso of utilizing CT for COVID-19 is that the explicitness is low (25%) in light of the fact that the imaging highlights cover with other viral pneumonia.
COVID-19 is at present determined to have RT-PCR and has been screened for with CT filters, yet every strategy has its own downsides. There are three issues that have emerged with RT-PCR. In the first place, the accessibility of PCR reagent packs has not stayed aware of interest. Second, people group emergency clinics outside of urban areas come up short on the PCR framework to suit high example throughput. In conclusion, RT-PCR depends on the nearness of recognizable SARS-CoV-2 in the example gathered. In the event that an asymptomatic patient was tainted with SARS-CoV-2 however has since recuperated, PCR would not recognize this earlier disease, and control measures would not be implemented. In the interim, CT systems are costly, require specialized mastery, and can’t explicitly analyze COVID-19. Different advances should be adjusted to SARS-CoV-2 to address these lacks.
Rising Analytic Tests for COVID-19
As per the WHO, the prompt need for COVID-19 diagnostics look into is the improvement of nucleic corrosive and protein tests and recognition at the purpose of-care.2 The more extended term need is to incorporate these tests into multiplex boards. So as to improve observation endeavors, serological tests utilizing proteins are required notwithstanding nucleic basic analyses. These tests have the advantages of discovery after recuperation, not at all like nucleic analyses. This empowers clinicians to follow both wiped out and recuperated patients, giving a superior gauge of all out SARS-CoV-2 contaminations. Purpose of-care tests are practical, hand-held gadgets used to analyze patients outside of brought together offices. These can be worked in territories like public venues to diminish the weight on clinical laboratories.
Protein Testing
Viral protein antigens and antibodies that are made in light of a SARS-CoV-2 disease can be utilized for diagnosing COVID-19. Changes in viral burden through the span of the disease may make viral proteins hard to recognize. For instance, Lung et al. demonstrated high salivary viral loads in the principal week after beginning of indications, which step by step declined with time. interestingly, antibodies created in light of viral proteins may give a bigger window of time for in a roundabout way distinguishing SARS-CoV-2. Immunizer tests can be especially helpful for reconnaissance of COVID-19. One potential test with creating exact serological tests incorporates potential cross-reactivity of SARS-CoV-2 antibodies with antibodies produced against different coronaviruses. Lv et al. tried plasma tests from 15 COVID-19 patients against the S protein of SARS-CoV-2 and SARS-CoV and saw a high recurrence of cross-reactivity.
At present, serological tests (i.e., blood tests for explicit antibodies) are in development. Zhang et al. recognized immunoglobulin G and M (IgG and IgM) from human serum of COVID-19 patients utilizing a compound connected immunosorbent examine (ELISA). They utilized the SARS-CoV-2 Rp3 nucleocapsid protein, which has 90% amino corrosive succession homology to different SARS-related infections. The recombinant proteins adsorb onto the outside of 96-well plates, and the overabundance protein is washed away. Weakened human serum is included for 1 h, after which the plate is washed once more. Antihuman IgG functionalized with horseradish peroxidase is added and permitted to tie to the objective. The plate is washed, trailed by the expansion of the substrate 3,3′,5,5′-tetramethylbenzidine. The peroxidase responds with the substrate to cause a shading change that can be distinguished by a plate peruser. In the event that enemy of SARS-CoV-2 IgG is available, it will be sandwiched between the adsorbed nucleoprotein and the antihuman IgG test, bringing about a positive sign. The IgM test by Zhang et al. has a comparative structure yet utilizes antihuman IgM adsorbed to the plate and an enemy of Rp3 nucleocapsid test. They tried 16 SARS-CoV-2 positive patient examples (affirmed by RT-PCR) and found the degrees of these antibodies expanded over the initial 5 days after side effect beginning. On day zero, half and 81% of patients were sure for IgM and IgG, individually, yet this expanded to 81% and 100% at day five. Antibodies were identified in respiratory, blood, or fecal examples. Xiang et al. likewise recognized SARS-CoV-2 IgG and IgM antibodies in suspected cases.Given the ongoing investigations, there may likewise be other protein or cellular markers that can be utilized for recognition. Guan et al. indicated that tainted patients had raised degrees of C-receptive protein and D-dimer just as low degrees of lymphocytes, leukocytes, and blood platelets.The test of utilizing these biomarkers is that they are likewise anomalous in different ailments. A multiplex test with both immune response and little atom markers could improve particularity.
Purpose of-Care Testing
Purpose of-care tests are utilized to analyze patients without sending tests to unified offices, subsequently empowering networks without research facility framework to identify contaminated patients. Sidelong stream antigen identification for SARS-CoV-2 is one purpose of-care approach being worked on for diagnosing COVID-19. In business horizontal stream measures, a paper-like film strip is covered with two lines: gold nanoparticle-neutralizer conjugates are available in one line and catch antibodies in the other. The patient’s example (e.g., blood and pee) is saved on the film, and the proteins are drawn over the strip by fine activity. As it passes the principal line, the antigens tie to the gold nanoparticle-neutralizer conjugates, and the perplexing streams together through the layer. As they arrive at the subsequent line, the complex is immobilized by the catch antibodies, and a red or blue line gets noticeable. Singular gold nanoparticles are red in shading, yet an answer containing grouped gold nanoparticles is blue because of the coupling of the plasmon band. The sidelong stream examine has exhibited a clinical affectability, particularity, and precision of 57%, 100%, and 69% for IgM and 81%, 100%, and 86% for IgG, individually. A test that identifies both IgM and IgG yields a clinical affectability of 82%.76 Nucleic corrosive testing can likewise be fused into the horizontal stream examine. Already, a RT-Light test was joined with sidelong stream readout to distinguish MERS-CoV. These tests are single utilize and experience the ill effects of poor explanatory affectability in contrast with RT-PCR. To improve the measure readout signal, analysts have built up an assortment of sign intensifying strategies (e.g., warm imaging and gathering of different gold nanoparticles).
Another methodology for use at the purpose of-care is microfluidic gadgets. These gadgets comprise of a palm-sized chip carved with micrometer-sized channels and response chambers. The chip blends and isolates fluid examples utilizing electrokinetic, slender, vacuum, as well as different powers. These chips can be built with materials, for example, polydimethyl sulfoxide, glass, or paper. The key focal points of utilizing microfluidics incorporate scaling down, little example volume, quick discovery times, and portability.80 Laksanasopin et al. built up a microfluidics-based cell phone connection to identify antibodies against three explicitly transmitted contaminations by consecutively moving reagents prestored on a tape. The stage demonstrated 100% and 87% clinical affectability and explicitness for HIV, individually, when tried on 96 patients in Rwanda.81 These advancements can be adjusted to recognize SARS-CoV-2 RNA or proteins.
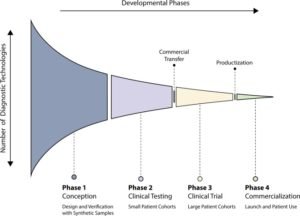
Here, we portrayed some indicative innovations that have demonstrated clinical possibility.There are numerous stages being created in scholarly research facilities, for example, electrochemical sensors, paper-based systems, and surface-upgraded Raman dissipating based systems. Such methodologies are in the beginning periods of advancement and can’t be utilized to analyze COVID-19 right away. One can outline analytic innovation improvement into four stages (Figure). Stage 1 alludes to advances that are at the proof-of-idea stage where scientists utilize engineered focuses to approve the idea. Stage 2 alludes to advancements that have examined few patient examples (i.e., <100 tests). Stage 3 commonly alludes to innovations that advance to clinical preliminaries with an enormous patient associate. Stage 4 is the point at which the innovation is marketed and utilized in patients. These developing innovations could assume a job in distinguishing future episodes.