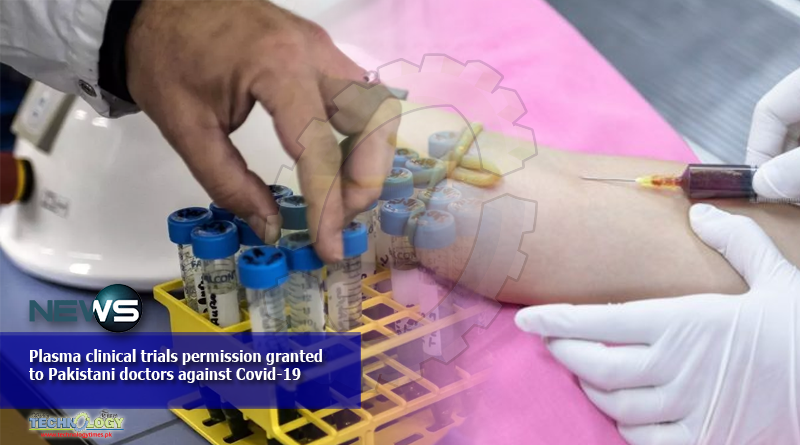
With the sharp ascent in cases, Pakistan is starting Plasma clinical trials of a possibly encouraging treatment to treat corona patients.
Pakistan’s Drug Regulatory Authority of Pakistan (DRAP) has endorsed Recuperating Plasma (CP) treatment for patients with genuine COVID-19 contaminations “subsequent to satisfying set guidelines of value and security” said DRAP CEO.
Pakistan’s National Institute of Blood Diseases and Bone Marrow Transplantation (NIBD) said that it has gotten official endorsement to lead clinical preliminaries for aloof inoculation. NIBD has additionally made sure about research award from pharmaceutical organization, Hilton Pharma, and different givers. NIBD’s hematologist Tahir Shamsi was one of the first to recommend the acquaintance of plasma treatment with the Pakistan government.
NIBD in Karachi is teaming up with the University of Health Sciences (Lahore), Liaquat University of Medical and Health Science (Jamshoro) and Sindh Blood Transfusion Authority, to present plasma-based medicines in Pakistan. Lahore University of Management Sciences (LUMS) Medical University in Lahore and Dow University of Health Sciences (DUHS) in Karachi are additionally forcefully seeking after plasma treatment under the supervision of specialists and researchers.
The treatment includes injecting antibody-rich plasma from recuperated COVID-19 patients into the individuals who are as yet tainted. Antibodies, otherwise called immunoglobulins, are proteins in the blood that battle explicit microorganisms and infections. The contributor antibodies briefly help debilitated individual battle contamination adequately.
Another group of Pakistani scientists from DUHS is certain that they are near one of the main affirmed plasma-determined treatment. The DUHS investigate group, drove by Dr. Shaukat Ali, has arranged Intravenous Immunoglobulin (IVIG) with plasma got from recuperated patients. “Along these lines of treatment is protected, okay and exceptionally viable against coronavirus,” as per an official articulation. “Through this strategy, immunoglobulin is set up after detachment of antibodies found in the blood of a recouped persistent.”
The DUHS specialists guarantee that what they have accomplished is “the main worldwide report of detachment, detailing and wellbeing exhibit of immunoglobulin decontaminated from recouped COVID-19 patients.” The group is now waiting to begin clinical trials.
Pakistani researchers state that their accomplishment is “a significant advance towards worldwide endeavors” to control and battle COVID-19. DUHS Vice-Chancellor considered it a huge “forward leap” and named it a “beam of expectation right now emergency”.
The US Food and Drug Administration has likewise permitted the utilization of plasma on COVID-19 patients in a crisis circumstance. Clinical trials for the plasma treatment are in progress in the US, UK, China, France, and Germany.
Dr. Faheem Younus, head of infectious diseases at the University of Maryland UCH, has named plasma treatment “extraordinary compared to other treatment choices accessible today” without immunization and rare assets, for example, test units, covers, and ventilators. Be that as it may, he depicted plasma, in his most recent article, as a “stopgap measure against the pandemic before we get to a viable antibody”