As the median age of the population increases, the number of individuals with Alzheimer disease (AD) is increasing and their condition is becoming worsen day by day.
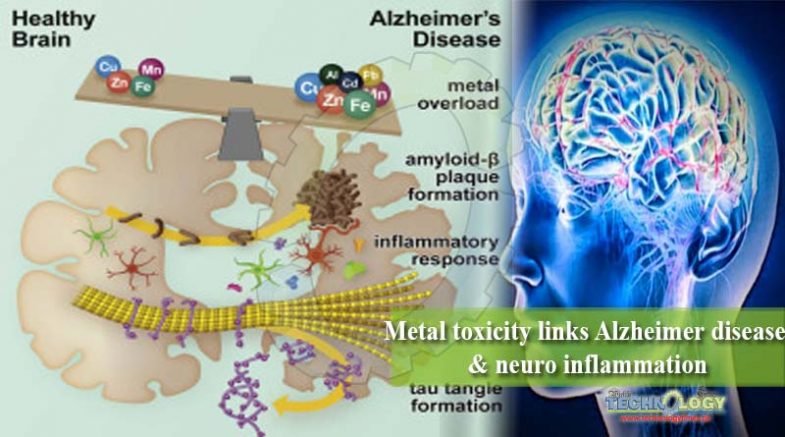 While aging and inheritance play major roles in the on set of Alzheimer disease, physical fitness, medical condition, and social environment have emerged as disease modifiers. Among known environmental risk factors, chronic exposure to various metals has become more common among the public. As a result, we are exposed not only to essential metals, such as iron, copper, zinc and manganese, but also to toxic metals including lead, aluminum, and cadmium, which perturb metal homeostasis at the cellular and organismal levels.
While aging and inheritance play major roles in the on set of Alzheimer disease, physical fitness, medical condition, and social environment have emerged as disease modifiers. Among known environmental risk factors, chronic exposure to various metals has become more common among the public. As a result, we are exposed not only to essential metals, such as iron, copper, zinc and manganese, but also to toxic metals including lead, aluminum, and cadmium, which perturb metal homeostasis at the cellular and organismal levels.
Herein, we review how these metals affect brain physiology and immunity, as well as their roles in the accumulation of toxic AD proteinaceous species (i.e., β-amyloid and tau). Our goal is to increase the awareness of metals as players in the onset and progression of Alzheimer disease.
Neuropathologically, AD is characterized by the misfolding and aggregation of two key proteins in the brain, Aβ and tau, leading to the formation of plaques and neurofibrillary tangles (NFTs), respectively. The formation of plaques is preceded by the production of Aβ, caused by changes in amyloid precursor protein (APP) processing, with NFTs forming due to abnormal hyperphosphorylation of tau.
APP is abundant in the healthy brain, where it is cleaved by α-secretase to produce the non-pathogenic protein fragment soluble APPα. However, in the AD brain, APP cleavage by β- and γ-secretase leads to the production of toxic Aβ fragments. These are prone to misfolding and forming oligomers, which are considered the most toxic Aβ species. Although it has been demonstrated that Aβ production and misfolding occur prior to any of the other pathologies associated with AD, the trigger(s) for this remains to be fully identified.
The production of Aβ has been shown to lead to the hyper phosphorylation of the microtubule-associated tau protein, stimulated by the phosphorylation of the tau kinases glycogen synthase kinase-3β (GSK-3β), cyclin-dependent kinase 5 (CDK5) and extracellular signal-regulated kinase. The hyper phosphorylation of tau can cause micro tubule destabilization and breakdown, perturb axonal transport, and make tau far more prone to aggregation into NFTs. NFT load correlates with cognitive impairment and neurodegeneration, leading to the suggestion that reducing tau hyper phosphorylation and NFT formation are key to preventing AD.
The brain has mechanisms to clear toxic proteins, including degradation pathways and the immune system. Inflammation poses a key risk for AD with respect to both genetic (e.g., apoe, trem2, cd33) and lifestyle factors (e.g., diabetes mellitus, obesity, traumatic brain injury). With respect to the proteins involved in AD, both Aβ and tau can be altered by neuro inflammation. Accumulation of Aβ triggers a pro inflammatory response from the brain’s resident immune cells, microglia and astrocytes, leading to the phagocytosis of plaques as well as their proteolytic degradation.
Receptors on microglia, such as triggering receptor expressed on myeloid cells 2 (TREM2) and toll-like receptors (TLRs), can recognize Aβ and trigger a phagocytic pathway. Aβ degrading enzymes, including neprilysin and insulin degrading enzyme, can also be released to remove Aβ extracellularly, although the activity of these enzymes is reduced in AD.
Moreover, the exacerbated pro inflammatory state that occurs during this period of the disease can trigger the hyper phosphorylation of tau. Several of the kinases responsible for tau phosphorylation are activated by pro inflammatory mediators and have been shown to worsen tau pathology. Interestingly, microglial uptake of hyperphosphorylated tau is linked to the propagation of tau pathology, and the over activation of pro inflammatory microglia by Aβ may therefore lead to worsened tauopathy.
Microglia become senescent over the progression of AD, in part due to the excessive production of Aβ. During this phenotypic state, they continue to produce microglia-recruiting, proinflammatory mediators, including cytokines and chemokines, causing more microglia eventually becoming senescent. Not only can this exacerbate Aβ and tau pathology, but microglia can become overactive in neurodegeneration, excessively pruning synapses and injuring neurons.
Among the chemical elements of relevance to humans, metals play a significant role in both health and disease. Metals are natural constituents of the Earth’s crust and are disseminated into the biosphere through human activities. These compounds display high stability, solubility in atmospheric precipitation, and ability to be absorbed by soil and living organisms, with human exposure soaring because of an exponential increase in their use in several industrial, agricultural, domestic and technological applications.
Common sources of metals are mining, tailings, industrial waste, agricultural runoff, paints, treated timber, aging water supply infrastructure, vehicle emissions, lead-acid batteries, fertilizers and microplastics. The main routes of human exposure include ingestion, inhalation and dermal contact. Physiologically, some metals are either essential nutrients (e.g., iron and zinc) or relatively harmless (e.g., ruthenium, silver, and indium), but even these can be toxic in larger amounts or certain forms.
This is because metals are usually essential components of larger biological molecules that can interact with or regulate the levels of relatively large numbers of other molecules. This means that the optimal physiological concentration range between deficiency and toxicity of metals is relatively small and needs to be tightly controlled. Importantly, tiny amounts of non-essential metals also promote severe toxicity as they inadvertently disrupt the physiological activity of essential metals.
Because of their high degree of toxicity, cadmium, lead and aluminum rank among the priority metals that are of public health significance. These metal ions are induced to cause organ damage, even at lower levels of exposure. Notably, evidence suggests that dysregulation in the homeostasis of essential metals and exposure to non-essential metals have a significant impact on the pathogenesis of Alzheimer disease.
