A coronavirus disease (COVID-19) vaccine tested for the first time in humans is safe and induces a rapid immune response, researchers at China’s CanSino Biologics Inc reported on Friday in The Lancet medical journal.
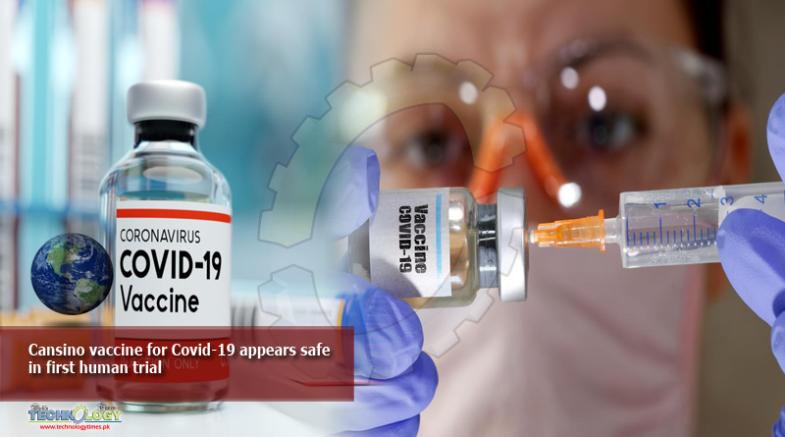
Blood samples from a group of 108 vaccinated adults showed both neutralizing antibodies and T-cell responses against the novel coronavirus in most of those tested.
Further studies will be needed to confirm whether the vaccine protects against infection.
“These results represent an important milestone. The trial demonstrates that a single dose of the new adenovirus type 5 vectored COVID-19 (Ad5-nCoV) vaccine produces virus-specific antibodies and T cells in 14 days, making it a potential candidate for further investigation,” coauthor Professor Wei Chen from the Beijing Institute of Biotechnology in Beijing said in a statement.
“However … the ability to trigger these immune responses does not necessarily indicate that the canSino vaccine will protect humans from COVID-19 … we are still a long way from this vaccine being available to all.”
This news was originally posted on gmanetwork.com
