In the 1930s, the first coronavirus was isolated in North Dakota, USA, from poultry suffering from a respiratory disease called infectious bronchitis.

Infectious bronchitis (IB) is a highly contagious and acute disease of poultry caused by the Infectious Bronchitis Virus (IBV). In the 1930s, the first coronavirus was isolated in North Dakota, USA, from poultry suffering from a respiratory disease called infectious bronchitis.
Particularly in high-density commercial manufacturing facilities, IBV continues to be a global concern for the poultry industry. With a high mortality rate (25–60%) in infected flocks, IBV has a significant economic impact. Chickens of all ages (young, layer, and broiler) are susceptible, with younger birds displaying more serious clinical signs than older ones. IBV infection is regarded as the second most threatening poultry illness in the world.
Etiology
Coronaviruses are the largest RNA viruses. They have an enveloped single-stranded RNA genome that ranges in size from 27 to 32 kb and a typical large club shape of 20 nm with highly glycosylated spike projections. The IBV genome contains four different genes that encode structural proteins. Spike (S), envelope (E), matrix (M), and nucleocapsid are the four types (N) (Fig. 1). A virus of the Coronaviridae family, genus Gammacoronavirus, and order Nidovirales.
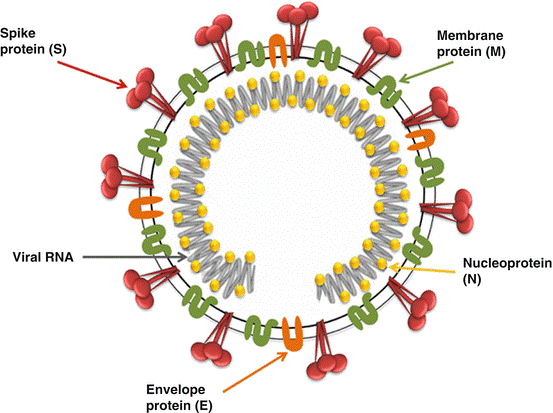
Function of Structural Proteins
The most abundant trans-membrane protein is M protein, which is essential for IBV assembly by interacting with spike glycoprotein as well as the viral ribonucleocapsid. E protein is involved in viral Ion channel activity, envelope formation, assembly, and budding, and apoptosis. IBV-N protein assists transcription, replication, translation, and packaging of the viral genome during replication and forms a helical ribonucleoprotein complex (RNP) with genomic RNA.
The S1 part of the spike protein is thought to be a determinant of viral diversity and immune protection because it plays a significant role in virus attachment and entry into the cell via sialic acid receptors. This protein has been identified as a candidate for recombinant IBV serotype vaccines, as well as genotypic characterization.
Transmission
The virus spreads through feces from infected poultry and respiratory secretions. Utensils and contaminated objects may aid in the spread. 24 hours after aerosol transmission, virus was found in the trachea, kidney, and Bursa of Fabricius.
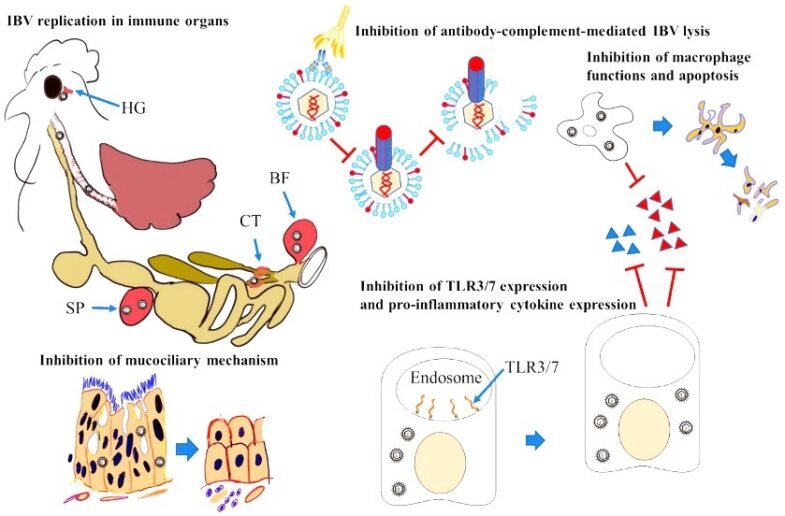
Immunosuppression Mechanism
IBV can stay in its host for a long time because it can copy itself in many immune organs. IBV replication mostly affects epithelial cells, causing them to lose their cilia and die. This stops the mucociliary escalation mechanism from working. Certain strains of IBV stop the expression of pathogen recognition receptors, such as TLRs, or their signaling pathways. This decreases the expression of proinflammatory cytokines, interferes with the innate immune system, and kicks off the adaptive immune system.
IBV can replicate in respiratory tract macrophages, impairing their functions and causing cell death. IBV can incorporate CD59 molecules into its envelope during entry and exit from host cells, protecting it from lysis through complement and antibody-dependent mechanisms (Fig.2).
Clinical sign and manifestations
The Hadrian gland, trachea, lungs, and air sacs are the first to be infected. The virus then spreads to the kidney and urogenital tract, infecting the internal organs. The organ or system affected by IB affects how severe it is and how it shows up in the body. Respiratory infections include gasping, sneezing, nasal discharges, tracheal rales, and listlessness (Fig.3A). Birds that are infected with IBV appear listless, dull, and have ruffled feathers. The huddling of birds around a common heat source and weight loss are two other signs.
IB infection can also cause frothy conjunctivitis, profuse lacrimation, oedema, and periorbital cellulitis. Infected birds are lethargic, with dyspnea and an aversion to movement (Fig.3B). Broiler-type chickens have been the most extensively studied for nephropathogenic IBV strains. Wet droppings, excessive water intake, and depression are all clinical signs.
The oviduct lesions, which result in decreased egg production and quality. Eggs may be misshapen, rough-shelled, or soft with watery yolks caused by the reproductive tract infection (Fig.3C).

Vaccination
Live and inactivated vaccines are used to protect chickens against IB. Outbreaks of IB are common in many poultry-producing countries, even after these countermeasures. This is most likely due to the emergence of new IBV variants. For long-lasting protection against IBV, humoral and cell-mediated immunity (CMI) must be activated.
In chickens experimentally challenged with IBV, the development of CMI has been linked to effectual clearance of virus, leading to depletion of clinical signs in sick birds. It has been known that clearance of IBV infected cells requires T cells based immunity and the increased presence of CD8+ T lymphocytes confers a good association with decreasing infection level and clinical signs in IBV infected chicken.
T Cells Based Immunity
T cell responses stimulated by vaccines are presently of great interest in mammalian research because they assure pathogen clearance at cellular level and a longer-lasting neutralizing antibodies for broader strain coverage. As a result, scientists shall be able to develop safer and more efficient T cell-based vaccines in the future for the poultry industry in Pakistan.
Flow Cytometry
While we understand cell – mediated immunity to pathogens in humans and mice, we know very little about cellular immunity to infectious diseases in birds. However, using immunological techniques that are extensively used in the studies of antigen presentation and T-cell functions in human and mouse research, the assessment of cell-mediated immune responses in birds after vaccination and viral infection has been noticed and is being improved through modified and substantiated flow cytometry.
Conclusion
It is critical to identify the prevalent IBV genotypes in the region, determine the cross-protective potential of commercially available vaccines, and optimize strategic immunization in-order to achieve effective protection against IBV.
This article is jointly written by Khadija Naseer,*1Muhammad Suleman1, Arfan Ahmad1, Haroon Akbar1 and Shah Jahan2 from 1University Diagnostic Laboratory, Institute of Microbiology, University of Veterinary and Animal Sciences, Lahore, Pakistan; 1Department of Parasitology, University of Veterinary and Animal Sciences, Lahore 54000, Pakistan; and 2University of health sciences, Lahore. The Corresponding Author* is Khadija Naseer.
