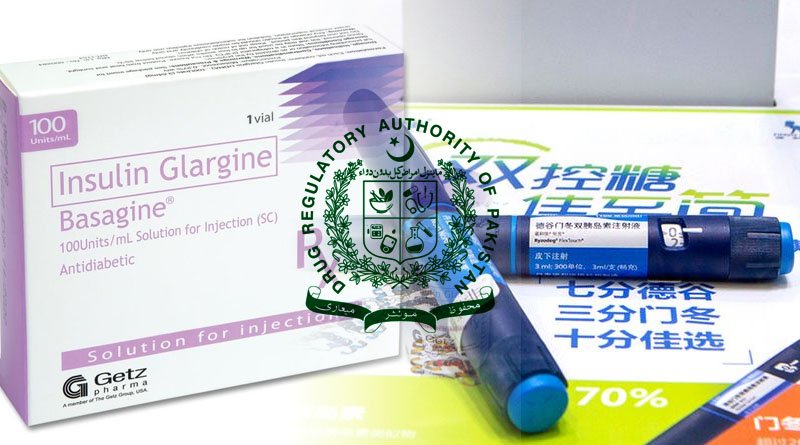
Insulin glargine injection is an essential long-acting insulin analogue product in the treatment of diabetes due to its efficacy and safety.
The Drug Regulatory Authority of Pakistan (DRAP) has approved the insulin glargine injectable Basagine developed by a Chinese pharmaceutical company for registration according to China Economic Net (CEN).
It is the first Chinese insulin glargine injection (prefilled injection pen) biosimilar drug in Pakistan, according to the company. Insulin glargine injection is an essential long-acting insulin analogue product in the treatment of diabetes due to its efficacy and safety.
In 2021, Pakistan had 33 million diabetics aged 20 to 79, ranking third in the world after China and India. According to the latest data from the International Diabetes Federation (IDF) Global Diabetes Overview, 10th Edition, the prevalence of diabetes among adults aged 20–79 in Pakistan is 30.8%, the highest in the world (2021).
It has been learned that the total market for insulin in Pakistan exceeds $50 million. Sanofi, Novo Nordisk, and Eli Lilly control the majority of the market in Pakistan, and the Chinese product will provide more high-quality options to Pakistani patients.
“The pharmaceutical trade between China and Pakistan has remained strong since 2010. We believe that as China relaxes its COVID restrictions, this momentum will continue to strengthen,” the company’s public relations department told CEN in an interview.
Meanwhile, Chinese patent medicines are being listed in Pakistan, promoting intensive collaboration, according to CEN. Lianhua Qingwen capsules, a Chinese patent medicine owned by Shijiazhuang Yiling Pharmaceutical Co., Ltd. (stock code 002603), have recently obtained permission to be listed in Pakistan.
Chinese insulin is the third patent medicine to be marketed in Pakistan, following the sale and use of Tai Ji Huoxiang Zhengqi oral liquid and Jian Zhi syrup.
Lianhua Qingwen capsules have been included in the diagnosis and treatment protocols or guidelines for infectious public health events such as influenza A, influenza B, and COVID-19 issued by China’s National Health Commission and the State Administration of Traditional Chinese Medicine for more than 30 times and have demonstrated unique value in the prevention and control of the epidemic.
Following registration approvals or import licences in nearly 30 countries and regions, including Canada, Thailand, and Singapore, the launch of Lianhua Qingwen in Pakistan is another overseas marketing licence.
Lianhua Qingwen capsules distributor, Pakistan Huazhilong International Trading Company, and Tai He Tai Law Firm collaborated for 31 months to apply for the Drug Regulatory Authority of Pakistan’s listing approval number.
The application process went extremely well for both the company team and the Chinese law firm. Cao Wei, CEO of Huazhilong International, said in a recent interview with China Economic Net (CEN) that “listing in Pakistan is of great significance to the internationalisation of Lianhua Qingwen and other Chinese patent medicines.”
Even the sale and use of Lianhua Qingwen in Pakistan have helped the country fight the pandemic. “In the future, China-Pakistan medicine collaboration could be expanded to treat common colds, various pathogens, hyperglycemia, hypertension, and other diseases.”
Tang Yunxia (also known as Donna Tang) of the Chinese law firm TAHOTA stated, “We can use these similar cases to help Chinese investors in the pharmaceutical industry invest in Pakistan, improve local capacity to produce medicines, and also export to Middle Eastern countries.”
The China-Pakistan Economic Corridor (CPEC) has entered its second phase of high-quality development, with an emphasis on improving cooperation in industries, science and technology, agriculture, and people’s livelihoods.
The construction of a health corridor is an important part of this process. Cao Wei believes that Pakistan is China’s all-weather strategic cooperative partner and that the two sides’ cooperation prospects are broad, with more Chinese enterprises willing to participate vigorously in the process.