Researchers have find out that blood sample from particular regions of genome can be accomplish to figure out epigenetic parameters in the body and showing a path to check epigenetic cause of diseases. Latest researches reveal that late-onset Alzheimer’s disease is driven by epigenetic changes.
By Duaa Azam and Aatika Shahid
Combating and resisting Diseases with Epigenetic Therapy
A disease is simply an abnormal condition that imparts negative effects on structures and functions of an organism. Humans have been facing various types of diseases worldwide. Scientist, doctors and researchers are working on their causes and prevention from years. Still causes of many diseases are unknown to them. It was observed that even after mapping of human genome, scientists were still unable to determine the causes of some diseases. They came to know that diseases cannot be predicted only on the basis of one’s genes. This leads the researchers to discover epigenetic cause of disease. But the progress has been slow as the study of epigenetics cannot be approached the same way as genetics, so it needs must more attention of scientists and researchers.
Epigenetic is the study of genetically changes in gene expression that are not engage with the change in DNA sequence. It is simply a change in phenotype without the change in genotype. This in turn affects how cells understand genes.
“Epigenetic is concern with the marking of DNA. It shows cells in the body which genes to switch on or off in that cell type. Cell-specific nature of epigenetics makes it difficult to study,” said Waterland, professor of pediatrics – nutrition and of molecular and human genetics.
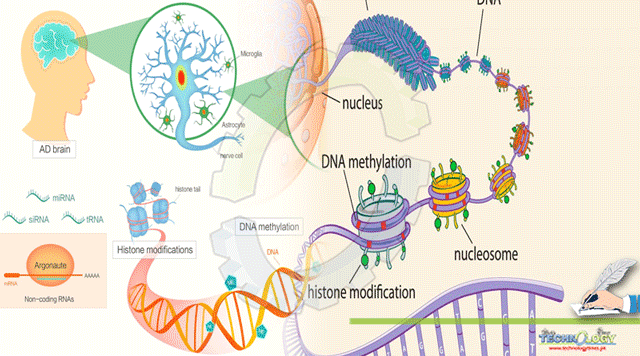
Recent studies reveal that a blood sample can be used to ‘genotype’ a person. Waterland and his co workers examined the regions of the genome that could be found in a blood sample and used to assume the epigenetic parameters throughout the body. This will allow scientists to check for epigenetic causes of a disease. For this they paid attention on the most stable form of epigenetic regulation i.e.; DNA methylation. This involves the additional of methyl groups to the DNA molecules in embryonic state. They portray DNA methylation all the way through the genome in three tissues i.e. thyroid, brain and heart from each of 10 cadavers to determine the genomic regions in which DNA methylation vary between different people but remain constant in different tissues.

“Latest studies disclose that methylation causes diseases like cancer, autism, obesity, cleft palate and Alzheimer’s disease.’’ said Dr. Cristian Coarfa, associate professor of molecular and cellular biology, the Dan L Duncan Comprehensive Cancer Center and the Center for Precision Environmental Health at Baylor, and co-leader of the project.
“Because epigenetic marking has the ability to stably silence or stably activate genes, any genetic disease could have an epigenetic origin,” Dr. Robert A. Waterland said.

Disclosing Epigenetic cause of Alzheimer’s disease:
Alzheimer’s disease also implies epigenetic changes. It is possible and logical to deal these modifications with epigenetic treatments. These changes are considered to be an ultimate target s they are reversible by nature, disparate DNA sequence mutation. Scientists are intended to amend either DNA methylation or histone acetylation.
“The last five years have seen huge hard work to widen therapeutics to treat Alzheimer’s disease, but they have failed in the clinic to treat humans facing this horrible disease,“ Berger said.
Researchers are trying their best to divulge the decisive changes in brain cells and their findings demonstrate that epigenetic changes are causing diseases. Epigenetic changes amend gene expression without DNA mutation, but slightly by marking proteins that wrap up and protect DNA, called histones.
Berger added, “The action of epigenetic regulators can be repressed by drugs, and hence we are eager that this may be an Achilles’ heel of Alzheimer’s that can be attacked by new therapeutics.“
The researchers incorporated a number of considerable advanced approaches of RNA, protein, and epigenomic analyses of postmortem human brains to cross-examine the molecular pathways concerned with Alzheimer’s. They found up regulation of transcription and chromatin associated genes, including of central histone acetyltransferases for marks that open up the chromatin (marks called acetylation of lysine 27 and 9 on histone H3, or H3K27ac and H3K9ac). Proteomic screening locates these marks as enriched in Alzheimer’s. The result were checked functionally in a fly Drosophila model to give an impression about that increasing these marks exacerbated Alzheimer’s disease associated effects.
“Based on our findings, there is a reconfiguration of the epigenomic landscape — that’s the DNA genome plus associated proteins — normally with age in the brain,“ Bonini said.
These changes do not arise in Alzheimer’s rather other changes observed. Surprisingly, the simple fruit fly Drosophila, in which Alzheimer’s-associated proteins can express and confer an Alzheimer’s effect, verify that certain types of mutations in the epigenome that can predict are related with Alzheimer’s intensify Alzheimer’s protein toxicity.
Conclusion:
‘‘We found in the earlier study that definite acetylation marks shield the brain during normal aging, while, unusually, in our innovative study, we found that other acetylation marks oblige disease. After that step is to recognize pathway underlying the protective and degradative mechanism, which will direct to a more under attack approach for Alzheimer’s disease therapy,“ Nativio said.
Authors: Duaa Azam and Aatika Shahid Students of Kinnaird College for Women University, Lahore. BS Biotechnology (Semester 3)