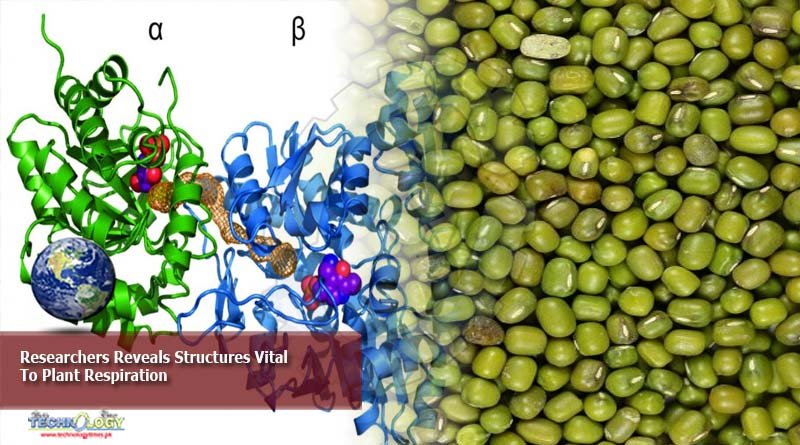
A new study provides the atomic-level 3D structure of the proteins behind plant respiration – the release of energy from food intake.
Researchers from the Department of Molecular and Cellular Biology at the University of California – Davis have provided the “first-ever, atomic level, 3D structure of the largest protein complex (complex I).” This protein complex is involved in the mitochondrial electron transport chain in plants.
Both animals and plants have a respiration process that allows them to release the energy from the food they eat. Observed at a cellular level, the exchange occurs in the mitochondria – membrane-bound cell organelles in charge of producing the chemical energy that fuels cellular processes. Acquiring a better understanding of how these processes differ between plants and animals could be used to improve agriculture.
“Plant respiration is a crucial process biologically for growth, for biomass accumulation,” said Maria Maldonado, a postdoc researcher and an author in the study. She added that crop growth is basically dependent on the plants’ ability to accumulate biomass, as well as the reactions between photosynthesis and respiration. Maldonado further compared that for other living beings, such as mammals and yeasts, their respective electron transport chain structures are available at higher resolutions. The same can be said with their supercomplexes, which are higher order organization of protein complexes. However, for plants, these structures remain largely unavailable prior to the UCDavis report.
“Lots of pesticides actually target the mitochondrial electron transport chain complexes of the pest,” said James Letts, assistant professor in UCDavis’ Department of Molecular and Cellular Biology, College of Biological Sciences. Letts further explained that by understanding these structures, scientists can pesticides or fungicides that protects the plant from invasive pests or fungi without harming the plant and later, the human who consumes it.
To better investigate how the plant mitochondrial electron transport chain works, researchers have identified the mung bean (Vigna radiata) as the optimal model system. The team cites a number of advantages for this decision:
The mung bean easily sprouts and can be harvested in six days.
It can be etiolated, grown without light, minimizing the growth of chloroplasts, which according to the researchers, will “contaminate the mitochondrial preparations.”
The age and growth conditions for mung beans can be experimentally controlled
Its mitochondrial content has been identified to be higher than other plant options used in previous, related studies.
Maldonado adds that plants have both mitochondria and chloroplasts, a plant-only organelle responsible for its green color. However, both organelles are reportedly similar in size and in certain physical properties. Afterwards, the UCDavis research team extracted mitochondrial samples from the mung beans. Through single-particle cryoelectron microscopy (cryoEM), the researchers were able to identify the structure of the protein complexes.
The procedure allowed researchers to observe how the building block proteins of complex I are assembled at an atomic level. They were also able to identify the difference between mung bean structures compared with their analogues in mammal, yeast, or bacterial cells.
Origina;;y published at science