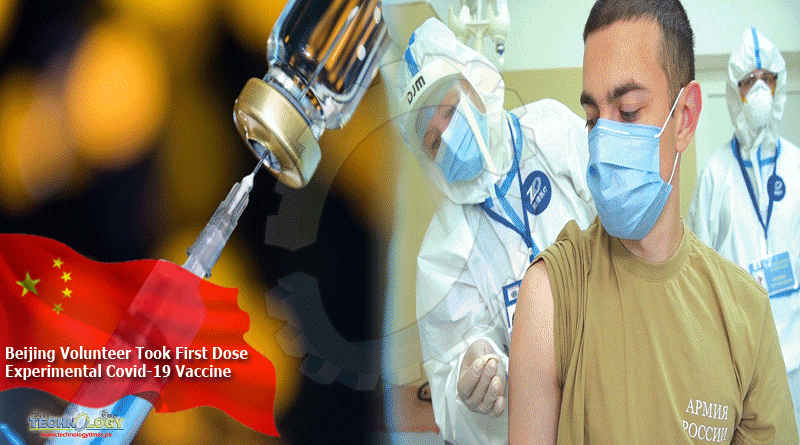
Three weeks ago, Beijing-based Wood Li took his first dose of an experimental Covid-19 vaccine.

Three weeks ago, Beijing-based Wood Li took his first dose of an experimental Covid-19 vaccine. At first, he hesitated because it has not yet been licensed for use on the market.
But the frequent business traveller eventually decided to take the chance, saying: “Since the company is offering it to people, it should not be a problem.”
Chinese media reports say increasing numbers have been vaccinated outside clinical trials although the exact numbers are not known.
In Li’s case he had to visit the headquarters of the China National Biotec Group, one of the country’s largest pharmacuetical firms, in Beijing and leave his contact details before returning for a second shot 28 days later. He said he had felt no ill effects after receiving the injection.
“Because it’s produced by CNBG, it should ensure the best quality in China,” Li said. “I saw there were dozens of people who came that morning and got the vaccine.”
Li said he had been given the jab for free because he had a friend in the health sector, adding that he thought the other recipients also had contacts in the industry.
Liu Jingzhen, chairman of Sinopharm, CNBG’s parent company, has previously said that he and other executives of the company had been the first to volunteer to receive the vaccine to verify its safety and effectiveness.
Gao Fu, director of China’s Centre for Disease Control and Prevention, also said recently that he had been vaccinated to build confidence for when mass vaccination is possible. He did not say which of China’s four experimental vaccines he had received.
On Friday, 21st Century Business Herald, a Chinese business newspaper, reported that some frequent business travellers were also being offered vaccines by an unnamed drug company on condition they signed an agreement not to tell others they had received the jab.
One businessman who took the vaccine told the newspaper that his employer had paid 1,000 yuan (US$143) but he did not know the reason for the charge.
Drug company employees told the newspaper that only those going abroad where transmission rates are high should be given the experimental vaccines.
Vaccines have to go through three stages of clinical trials before applying for a licence though regulators sometimes allow emergency exemptions.
China has not formally announced the emergency use of vaccines, although CDC chief Gao has this would be available in summer.
Liu Ye, a Shanghai-based lawyer practising in the medical field, said state-approved vaccines can also be offered to volunteers during the experimental process but must follow the demands of clinical trials.
“There must be a very strict informed consent agreement. And the volunteers should also be subsidised, it’s never allowed to charge volunteers for it,” said Liu.
Liu said the report that businesses were paying a 1,000 yuan fee was probably to cover insurance costs, but added that this cost should really be borne by the drug company.
The use of vaccines outside trials by Chinese pharmaceutical firms has raised eyebrows from some scientists, including Anthony Fauci, the director of the US National Institute of Allergy and Infectious Diseases.
First Dose, Two of Sinopharm’s vaccines are still undergoing phase three trials in the United Arab Emirates as transmissions in China are too low for extensive testing.
Another vaccine co-developed by military scientist Chen Wei and CanSino has been approved for military use, while phase three trials are taking place in Saudi Arabia.
Last week, CNBG and Sinovac, another vaccine producer, warned people not to fall for online scams in which advertisers are offering Covid-19 vaccines for sale.
This news was originally published at scmp.com