To learn periodic table is a bit difficult thing but now it is instantly recognizable. Periodic table found everywhere in Chemistry world.
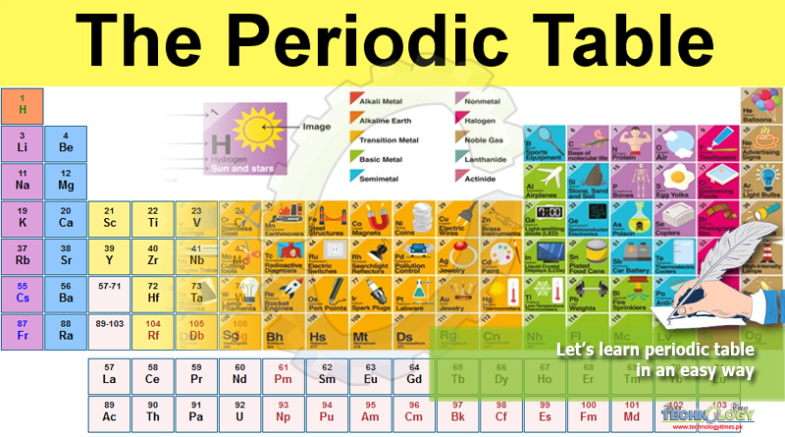
This well-known chart is found in chemistry textbooks and on classroom walls. It lays out all the known elements and has been around in various forms for more than 150 years.
This well-known chart is found in chemistry textbooks and on classroom walls. It lays out all the known elements and has been around in various forms for more than 150 years, but now it is easy to learn periodic table.
The notable outline of elements is found in science course books and on homeroom dividers. It spreads out all the known components and has been around in different structures for over 150 years. Be that as it may, the rendition of that diagram that Russian researcher Dmitri Mendeleev developed in 1869 isn’t a similar outline we see today.
In those days, his diagram contained just 63 components. They were all that was known. Mendeleev left holes in his table where anticipated, yet not yet known, components should fit. What’s more, when researchers discovered all the normally happening ones, they began assaulting two components together to make new components. Today, the table has 118 known components.
Seeing chemical elements arranged in the modern periodic table is as familiar as seeing a map of the world, but it was not always so obvious.
The creator of the periodic table, Dmitri Mendeleev, in 1869 began collecting and sorting known properties of elements like he was playing a game while traveling by train. He noticed that there were groups of elements that exhibited similar properties, but he also noticed that there were plenty of exceptions to the emerging patterns.
Incredibly, instead of giving up, he tried altering the measured property values to better fit the patterns! He also predicted that certain elements must exist which didn’t at the time – again, in an effort to get the patterns in his “game” to work out.
There were plenty of skeptics and it took years to gain international acceptance, but once newly-discovered elements matched the ones that Mendeleev predicted, his patterns could not be dismissed. In addition, some of the properties that he “fudged” were later recalculated and found to be much closer to his predictions.
