Phage Game: Apocalypse caused by super resistant bacteria seems quite unimaginable. But as a matter of fact the war between humans and bacteria is a tale as old as time.
 So far humans had the winning streak against bacteria due to the discovery and extensive use of antibiotics. Hence keeping the Pandora’s Box shut. But as the utilization of antibiotics grew so did the survival pressure on the bacterial population. Over and misuse of antibiotics led to mutations within the bacteria causing them to develop into resistant strains.
So far humans had the winning streak against bacteria due to the discovery and extensive use of antibiotics. Hence keeping the Pandora’s Box shut. But as the utilization of antibiotics grew so did the survival pressure on the bacterial population. Over and misuse of antibiotics led to mutations within the bacteria causing them to develop into resistant strains.
Such strains are drastically becoming immune to majority of our antibiotic arsenal. The champions of the bacterial offense are those resistant strains that have acquired complete resistance against all known antibiotics like Methicillin-resistant Staphylococcus aureus (MRSA) and Vancomycin-resistant Enterococcus (VRE).
However, all is not lost. Humans have allied with longtime rivals of bacteria, the “Virus”. Phages or bacteriophages are viruses that specifically infect bacteria. They hijack the host machinery to manufacture more virus particles and eventually end up killing their bacterial host.
Since we are running out effective antibiotic options, researchers are focusing on finding alternative therapeutic schemes and bacteriophages are a promising contender.
On 25th July 2019, Zeinab Hosseinidoust and her team published their work on self-healing, bioactive hydrogels with self-organizing phage nanofilaments in Chemistry of Materials. The research was first of its kind as it investigated properties other than biorecognition.
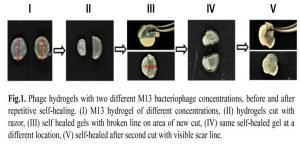
Her team propagated and extracted a bacteriophage called M13 from Escherichia coli (E.coli). The extracted bacteriophages were crosslinked with a glutaraldehyde (GA), a nonresponsive crosslinker, to form M13 hydrogels. The M13 self-organized at high concentrations with the help of their nanofilaments.
A yellow hue colored gel was produced that exhibited repetitive self-healing property at room temperature. Hosseinidoust and her colleagues incubated the hydrogel at room temperature in presence of calcium chloride (physiological levels) and then made an incision with a razor blade.
The gel exhibited self-healing process similar to biological tissues where healing could autonomously be trigger after damage (Fig.1). They then incubated the hydrogel in E.coli culture to check bioactivity.
A high titer of phage was seen in the culture which indicated that despite the M13 being encapsulated and fixed in a matrix, the phages were biologically active.
They were able to infect and propagate themselves in the bacterial host. This antibacterial hydrogel has the potential to be used as sterile growth scaffold in engineered tissues, coating on artificial implants and can also help in environmental cleanups.
Utilization of phages to fight bacterial infection has many advantages over antibiotics. Antibiotics are indiscriminate killers while phages are highly specific in their nature. This means that phages can be engineered to specifically attack harmful bacteria while not disturbing the harmonically symbiotic bacteria like those present in our gut.
Furthermore, bacteriophages can also kill antibiotic resistant superbugs. It is much harder for bacteria to develop resistance against phages. This is because instead of blocking one specific process like cell wall or membrane synthesis in case of antibiotics, phages actively destroy these functions simultaneously.
Phages are now being engineered to bring back a century old therapeutic called phage therapy. Phage therapy has successfully been used on a handful of patients suffering from cystic fibrosis that have developed widespread bacterial infection. However, much research is still need for extensive use of phage therapy.
The post-antibiotic era is upon us and we are inefficiently equipped for the battles that lie ahead. Work similar to that of Zeinab Hosseinidoust and her team can provide potential solutions against ever evolving bacterial resistance and will keep us from reverting back to pre-penicillin age.
Authors: Nimra Khurram and Muhammad Mustafa